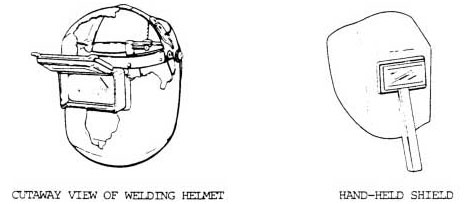
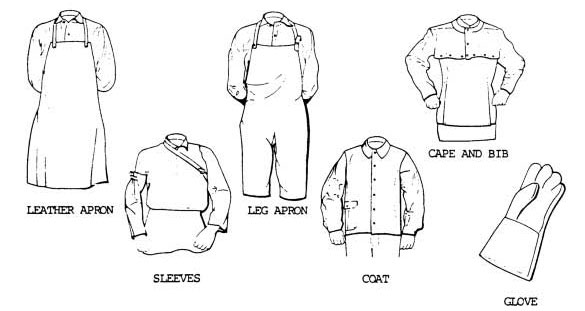
Acetylene is commonly used for fueling cutting torches in both general industry and the mining industry. As the gas is highly combustible when comes in contact with the oxygen, it is recommended to ensure safety measures to avoid accidents.
Chemical Composition: An acetylene molecule is fabricated by the composition of two carbon atoms and two hydrogen atoms. In fact, the two carbon atoms are incorporated together, which is known as a triple carbon bond. This bond is vital as it stores substantial energy that can be released as heat during combustion. On the other hand, the triple carbon bond is unstable, making acetylene gas very sensitive to a number of conditions such as excess pressure, excess temperature, static electricity, or mechanical shock.
Working with acetylene gas requires specialized equipment and materials. Acetylene cylinders are specially designed and developed for storage of the gas. The compressed gas cylinders contain gas stored under significant pressure, posing a significant hazard in the workplace. Some of the hazards due to the chemical properties of gas cylinders include:

Storage: Acetylene gas being unstable in nature, it requires to be stored under special conditions. It should be stored by dissolving the acetylene in liquid acetone. Then, liquid acetone is stored in acetylene cylinder which is filled with a porous material.
Acetylene plants are specially designed and manufacture taking proper precautions to prevent fire and explosions. Acetylene is a highly flammable gas so it should be kept away for oxygen.

Copyright © 2023 acetyleneplant.net All Rights Reserved.

